ADP Diagnostics is an innovative diagnostics company based in Carrickfergus, Northern Ireland, supplying products to the Clinical Diagnostic Laboratories in both the public and private sectors.
As a science graduate of Ulster University and now with 25 years experience in clinical diagnostics, my goal for ADP Diagnostics is to make it a world leader in supplying speciality quality controls, Drugs of Abuse testing kits and POCT equipment. We work with carefully chosen manufacturers and strive to provide bespoke solutions for our customers. Research & Development, validations and trials are carried out in our own laboratory. The excellent laboratories in our local Belfast hospitals also provide invaluable validation data for our clinical products.
ADP Diagnostics is an ISO 9001:2015 registered company, recognising the quality of our administrative and management systems.
Greg Simpson Bsc.
Managing Director

| TOX-SCREEN 14 PANEL DRUG TEST SYSTEM | ||
| UCP-100 DRUG CUP SYSTEM |
|
Sample collection urine cup Unique 16 Panel 10 minutes to test results Accurate, Precise, Reproducible results On board quality control |
| DIP-CARD URINE TEST |
|
Rapid Test Dip-card Unique 16 Panel Fast. Test results in 10 minutes Easy. 3 step operation Minimal training required |
| UNIQUE 14 PANEL | AMP, BAR, BZO, COC, EDDP, FEN, K2, KET, THC, mAMP, MDMA, OPI, TRA, TCA, GAB, PGB | MHRA REGISTERED |
| FAST AND RELIABLE | SIMPLE SAMPLE PROTOCOL | AVAILABLE NOW |
20 January 2017
ADP Diagnostics are pleased to announce the appointment of AKSA Medical to represent us in the Benelux countries,
AKSA Medical
Kasper Scholte Albers
Spoorakkerweg 11
5071NC Udenhout
The Netherlands
Tel: +31 (0) 13 590 58 00 Fax: +31 (0) 13 590 57 44
Mobile: +31 (0) 6 305 320 86
28 October 2016
Every day in hospital laboratories across the world, millions of patient samples are being scanned on automated analysers for compromised samples. The ‘flag’ rate for compromised samples from Serum Indices (Haemolysis, Icterus and Lipaemia) can be up to 10%. However, how do we know that the automated analysers are accurately scanning the samples for Serum Indices?
Until recently, there has been no way to routinely check the indices-scanning performance of automated analysers. As well as this, developmental trials have shown differences of up to 10% between ‘analytical lines’. One analyser can exclude 10% more results than another analyser even in the same laboratory.
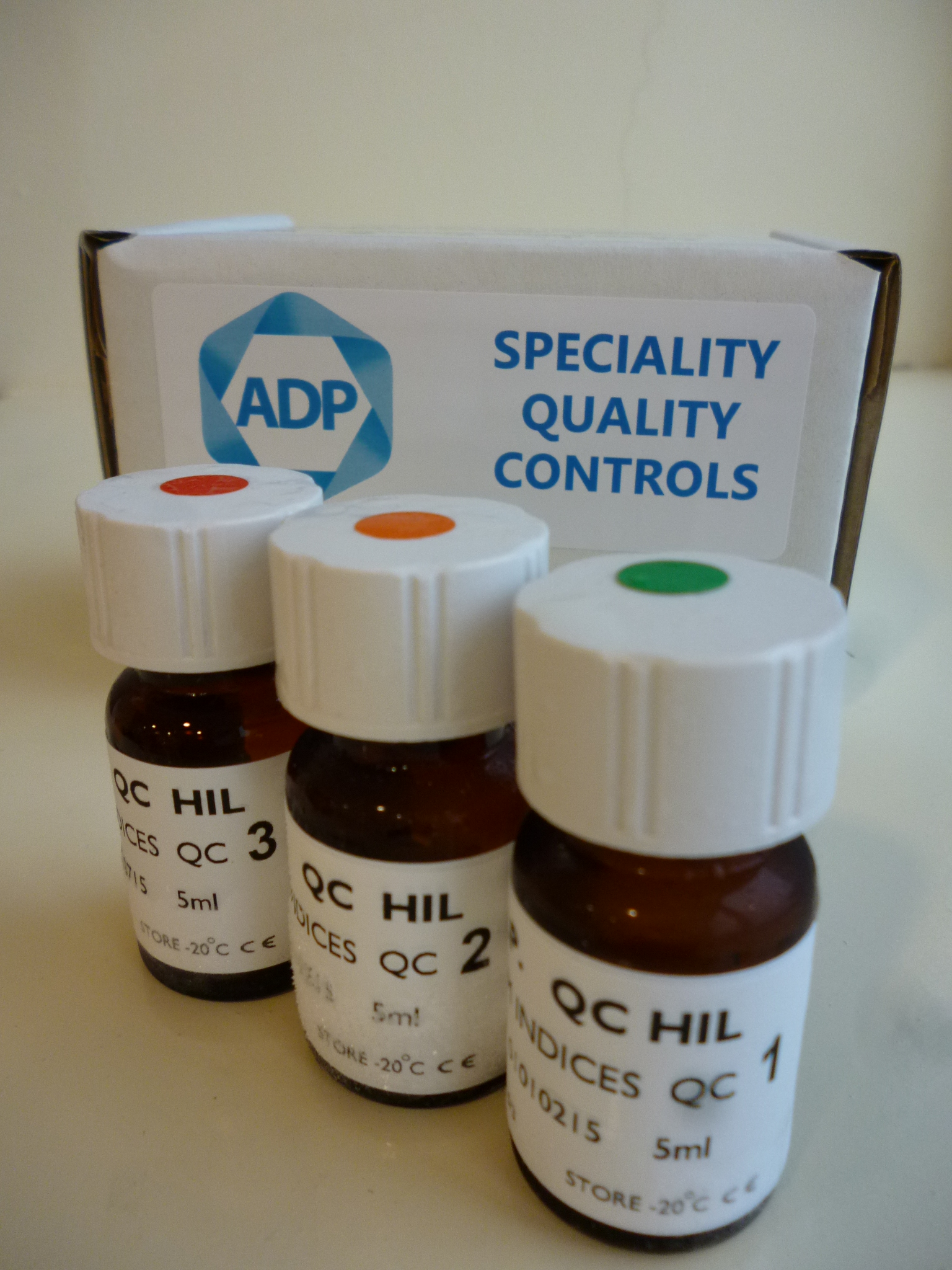
ADP Diagnostics has developed the world’s first Serum Indices Quality Control material to allow laboratories to check that their analysers are accurately scanning and flagging compromised samples.
The Serum Indices Quality Control is available as a frozen liquid stable product with all 3 parameters combined into one control. The control is available in 3 levels, 1+, 2+ and 3+. We have developed instrument specific controls suitable for Roche, Abbott and Beckman analysers. Pack size is 4 x 3 levels x 5ml.
Our Serum Indices Quality Control is the only 3rd party QC sera available on the market and is therefore essential for any laboratory that needs to comply with national and international standards, such as ISO 15189 accreditation.
The product is currently being used by hospital laboratories throughout the UK and Ireland, Europe and Australia.
ADP Diagnostics will have the official international launch of the Serum Indices Quality Control sera at Medica 2016 in November where we will be participating as an exhibitor. For more information on the product and market feedback, please visit our stand in Hall 1 F07.